Wherever you are in your product development lifecycle,
Sterling can help.
P: 201-877-5682
F: 201-301-9169
info@sterlingmedicaldevices.com
At Sterling Medical Devices, we provide a wide range of medical device services to help take your ideas for medical device projects from concept to market. Below are some of our past medical device projects to illustrate the scope of work we can provide while respecting the privacy and confidentiality of our clients. If you are looking for more in-depth examples of our design and development work, please review our case studies.










July 14, 2023

February 18, 2021
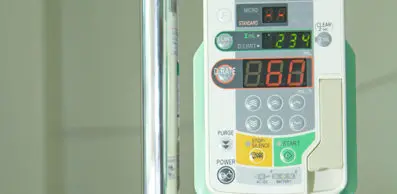
September 8, 2020